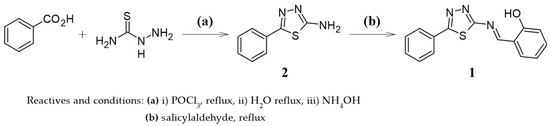
Aluminum (Al) and zinc (Zn) are two of the most widely used metals in industry, and their excessive accumulation in the body has been linked to serious diseases like Alzheimer’s, Parkinson’s, and cancer. This highlights the need for effective ways to detect and measure them. In this study, we synthesized the fluorescent chemosensor 1, which contains a Schiff base and a 1,3,4-thiadiazole ring in its structure, and evaluated its fluorescent response in the presence of various metal ions. The chemosensor enabled the selective quantification of Al3+ and Zn2+ ions through excitations at different wavelengths, yielding differentiated fluorescent emissions. For Al3+, excitation at 370 nm generated a strong emission at 480 nm, whereas for Zn2+, excitation at 320 nm led to a new small broad emission at 560 nm. We established detection limits of 2.22 × 10−6 M for Al3+ and 1.62 × 10−5 M for Zn2+; their binding stoichiometry was found to be 1:1 for Al3+ and 2:1 for Zn2+, based on Job’s plot analysis. These results show that chemosensor 1 is a promising tool for detecting Al3+ and Zn2+.