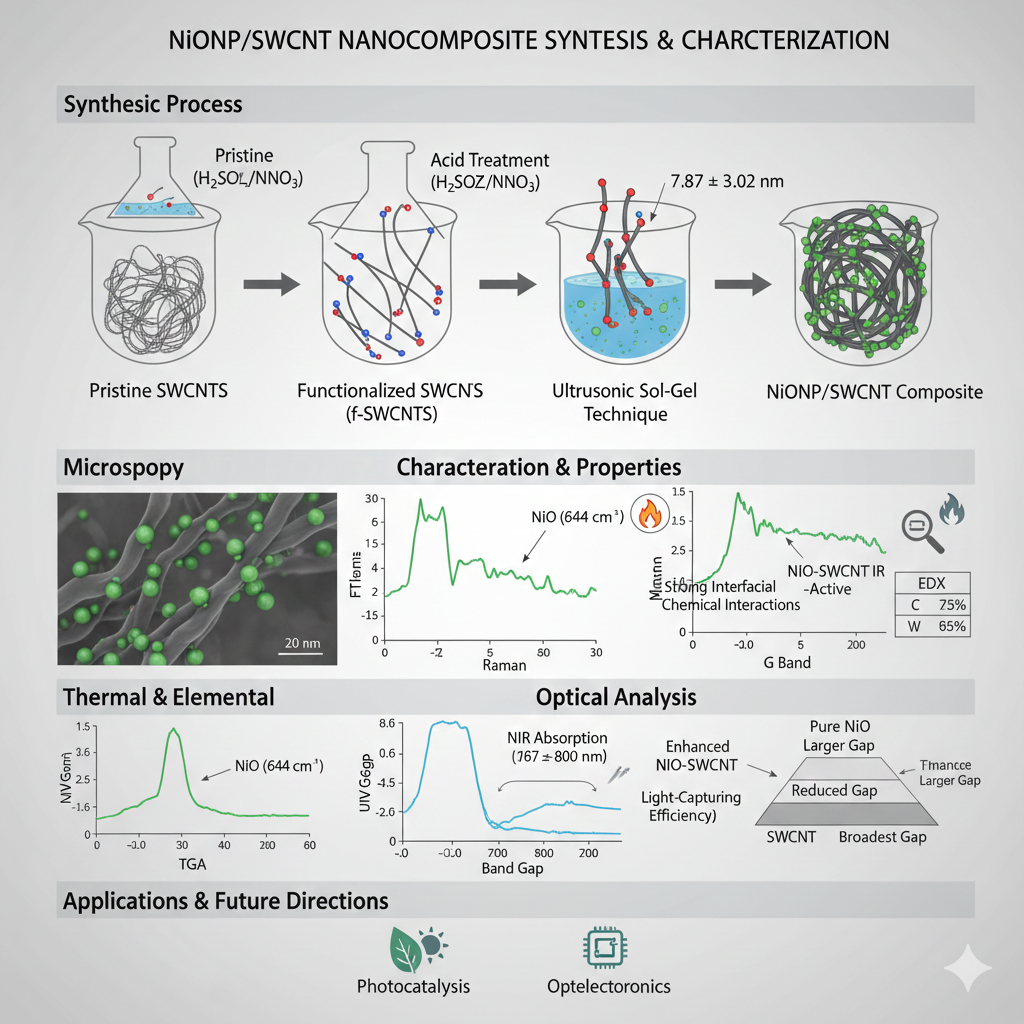
The decoration of carbon nanotubes with metal oxide nanoparticles has been employed to enhance their intrinsic properties and expand their applicability across various technological fields. This study investigated the functionalization of single-walled carbon nanotubes (SWCNTs) by treating them with a 3:2 mixture of sulfuric acid and nitric acid, which introduces oxygen-containing functional groups to enhance their dispersibility and reactivity. Nickel oxide nanoparticles (NiONPs) were synthesized and integrated onto the functionalized SWCNTs using an ultrasonic-assisted sol-gel technique, allowing uniform distribution.
Then, the NiONP/SWCNT composite was evaluated for thermal stability and elemental composition via thermogravimetric analysis (TGA) and energy-dispersive X-ray spectroscopy. Both field emission scanning electron microscopy and high-resolution transmission electron microscopy confirmed the successful decoration of NiONPs (particle size <20 nm, mean value of 7.87 ± 3.02 nm) on the SWCNTs. Fourier-transform infrared spectroscopy revealed characteristic peaks corresponding to NiO at 644 cm−1 as IR-active modes induced by NiO–SWCNT and Raman spectroscopy further verified the chemical bonding between NiONPs and SWCNTs.
This shows shifts in the radial breathing mode and G bands of SWCNTs, indicative of strong interfacial chemical interactions. Optical analysis demonstrated that the NiO-SWCNT nanocomposite exhibited a reduced band gap compared to pure NiO nanoparticles but a broader band gap than intermediate-phase SWCNT configurations. In addition, UV–Vis spectroscopy identified a prominent absorption peak within the 600–800 nm wavelength range, aligning with the near-infrared (NIR) spectral region. This enhanced NIR absorption suggests improved light-capturing efficiency, which could significantly benefit applications in photocatalysis and optoelectronics.